A resource for patients prescribed
IDACIO▼ (adalimumab) and their
healthcare professionals
The information on this page is intended for healthcare professionals only
Click below to register for access to the healthcare professional pages.
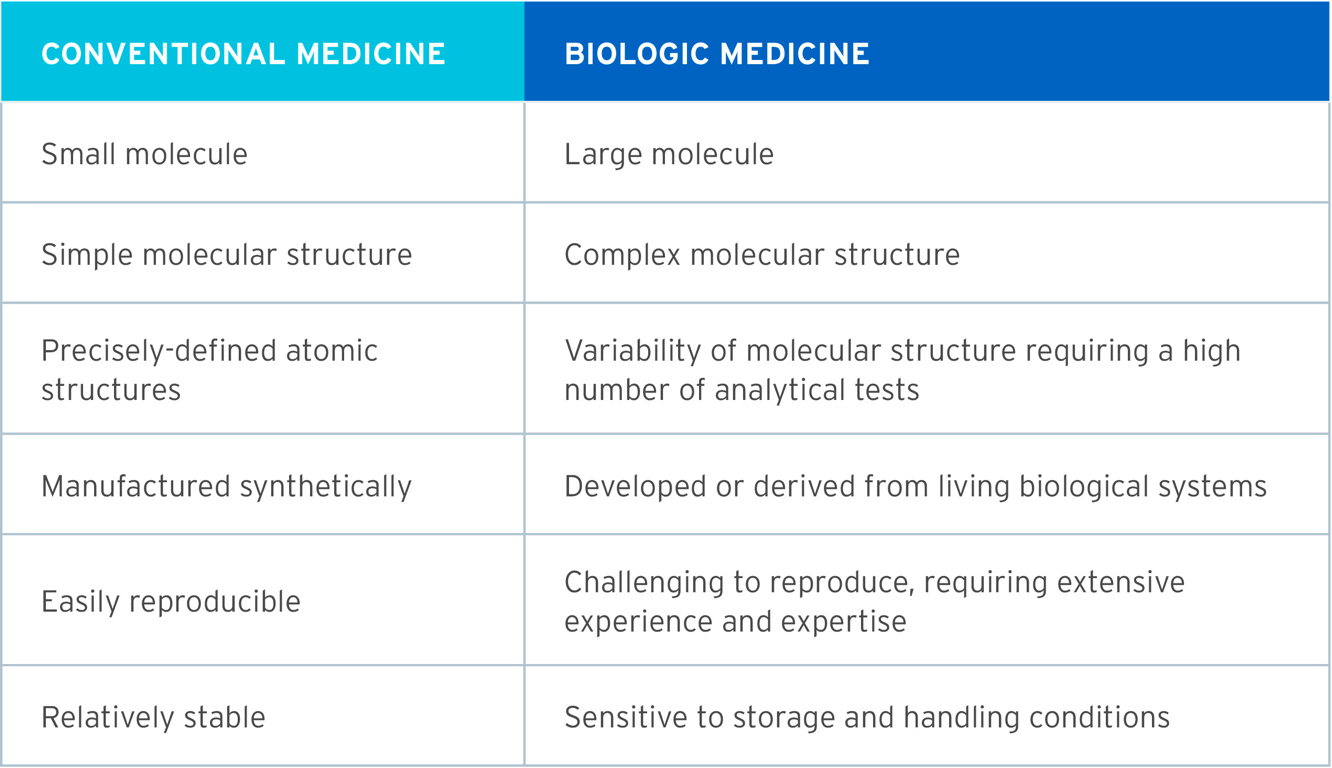
Biologic medicines have had considerable impact on the treatment of a range of chronic illnesses and life-threatening diseases.1 Biologics are large complex molecular proteins developed or derived from living biological systems, that utilise state-of-the-art biotechnology.1
The table summarises some of the differences between the development of a conventional medicine (e.g. aspirin) and a biologic medicine (e.g. adalimumab).1-4
A biosimilar is defined by the European Medicines Agency as 'a medicine highly similar to another biological medicine already marketed in the EU (the so-called 'reference medicine')'.4 Biosimilars are not the same as generic drugs.4 A generic drug is the same as a reference product in all aspects including quality and performance characteristics, since it is built by chemical synthesis.4 A biosimilar is highly similar, though not identical, to the reference product mainly because it is produced in living cells.4
Once the period of market protection of the reference medicine has expired, pharmaceutical companies can develop and market biosimilars.4 Healthcare systems are increasingly keen to use biosimilars that have demonstrated equivalent clinical efficacy and similar safety profiles to the reference product.4
Biosimilars undergo rigorous testing and must demonstrate that they are similar to the reference product through three different types of studies:4
Comparative quality studies: These in-vitro studies compare the protein structure and biological function of the biosimilar and the reference product.
Comparative non-clinical studies: these include pharmacodynamic studies (generally in vitro) to compare the biosimilar and reference product. In-vivo toxicological studies are required in certain cases.
Comparative clinical studies: Clinical trials are tailored to confirm biosimilarity and include studies of pharmacokinetics in healthy volunteers, or patients, and clinical studies performed to demonstrate comparative efficacy, safety and immunogenicity.
The EU regulatory guidelines for approval of biosimilars allow for extrapolation of data.4 This means that all the safety and efficacy data for the biosimilar and the reference product in one indication may be utilised to obtain approval for the use of the biosimilar in other approved indications of the reference product.4
Safety is a critical component of the approval of a new biosimilar, which has to demonstrate no clinically meaningful differences in immune response compared to the reference product. Immune responses may affect the safety or effectiveness of the product. In some cases, a dedicated immunogenicity study may be necessary.
This resource is directed at healthcare professionals who are treating patients with IDACIO.
1. Abraham J. Developing oncology biosimilars: An essential approach for the future. Semin Oncol 2013;40(Suppl1):S5-S24
2. Kozlowski S, Woodcock J, Midthun K, Sherman RB. Developing the Nation’s Biosimilar Program. N Engl J Med 2011;365:385-388
3. Medicines for Europe. Biosimilar Medicines Handbook. 2016 [online]. Available at: http://www.medicinesforeurope.... [Accessed May 2020]
4. Biosimilars in the EU. Information guide for healthcare professionals. European Medicines Agency. 2019 [online]. Available at: https://www.ema.europa.eu/docu... [Accessed May 2020]
Once registered, use the fields below to log in.
You are leaving the KabiCare website now. Please note that Fresenius Kabi have no affiliation or control over the content available on external third-party websites.
Continue